Ancient electrical cells have been discovered in Sumerian ruins dating from around 250 BC. The first evidence of batteries comes from archaeological digs in Baghdad, Iraq. One of the first uses for batteries was to electroplate objects with a thin layer of metal, much like the process used now to plate inexpensive gold and silver jewellery. The early jar cells were found in Khujut Rabu just outside Baghdad and is composed of a clay jar with a stopper made of asphalt. Sticking through the asphalt is an iron rod surrounded by a copper cylinder. When filled with vinegar - or any other electrolytic solution - the jar produces about 1.1 volts.

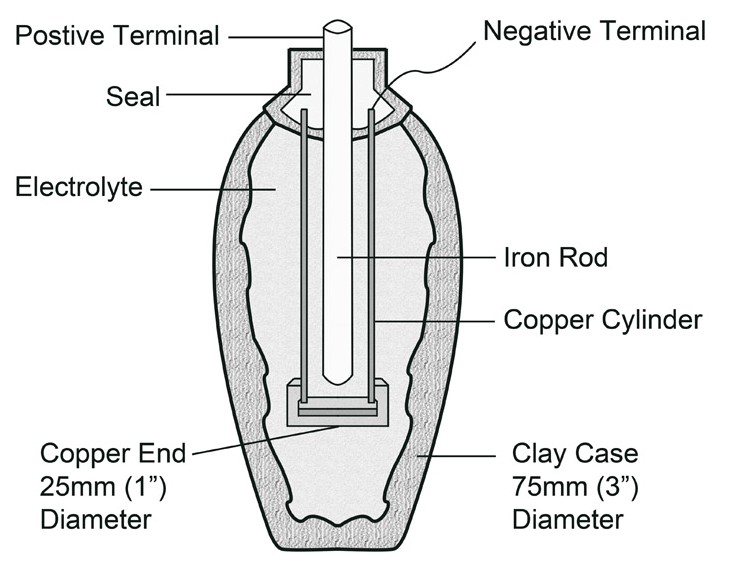
Baghdad Battery
Modern battery development dates as far back as the late 18th century. The cause was championed by the work carried out by Luigi Galvani from 1780 to 1786. Through his experiments Galvani observed that, when connected pieces of iron and brass were applied to frog's legs, they caused them to twitch. However, Galvani thought that the effect originated in the leg tissue. Nevertheless, Galvani had laid the cornerstone for further developments in "voltaic" electricity.

Leyden Jars
From 1796 - 1799, Alessandro Volta experimented with zinc and silver plates to produce electric currents at the Pavia University. Volta stacked the two to form a "pile", the first "dry" battery. By 1800 Volta had created the "crown of cups", a modified arrangement of zinc and silver discs dipped in a salt solution.

Alessandro Volta
In the years that followed other means of producing electricity were invented, all of which involved the use of liquid electrodes. Those developed by Bunsen (1842) and Grove (1839) were amongst the most successful systems, and, were used for many years.
By 1866, Georges Leclanche, a French engineer, patented a new system, which was immediately successful. In the space of two years, twenty thousand of his cells were being used in the telegraph system. Leclanche's original cell was assembled in a porous pot. The positive electrode consisted of crushed manganese dioxide with a little carbon mixed in. The negative pole was a zinc rod. The cathode was packed into the pot, and a carbon rod was inserted to act as a currency collector. The anode or zinc rod and the pot were then immersed in an ammonium chloride solution. The liquid acted as the electrolyte, readily seeping through the porous cup and making contact with the cathode material. Leclanche's "wet" cell (as it was popularly referred to) became the forerunner to the world's first widely used battery, the zinc carbon cell.

Plante Battery
Leclanche's invention, which was quite heavy and prone to breakage, was steadily improved over the years. The idea of encapsulating both the negative electrode and porous pot into a zinc cup was first patented by J.A. Thiebaut in 1881. But, it was Carl Gassner of Mainz who is credited as constructing the first commercially successful "dry" cell. Variations followed. By 1889 there were at least six well-known dry batteries in circulation. Later battery manufacturing produced smaller, lighter batteries, and the application of the tungsten filament in 1909 created the impetus to develop batteries for use in torches.
The production of batteries was greatly increased during the First World War as a means of powering torches, field radios. Other milestones in battery production include the widespread radio broadcasting, which brought battery-operated wireless into the heart of many homes. But, it was during the inter-war years that battery performance was greatly enhanced. This was achieved through better selection of materials and methods of manufacture.
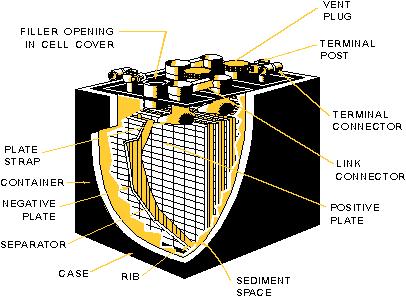
Lead-acid Battery Assembly
Batteries have now become an essential part of everyday life. They are the power source for millions of consumer, business, medical, military and industrial appliances worldwide. This demand is growing. The term 'battery' comes from having a number (a battery) of separate cell cases all joined together by electrical connections. These days the electrical connections and cells are normally included in one casing, such as in a car battery - in this case six 2 volt lead-acid cells totalling 12 volts.
There are many types of battery but they all store energy as chemical potential - i.e. two chemicals which react in a solution when a circuit is made. There are primary and secondary cells. Primary cells use the chemical components of the battery itself to produce energy (this includes fuel cells). When the components are used the battery is discarded, except in a fuel cell where the fuel spent can be replaced. Secondary cells, or accumulators may be re-charged many times over.
The most common type of secondary cell is the "lead-acid" type used to start motor car engines. Taken to the extreme lead-acid batteries can produce a very high output for a short duration. These batteries are the least expensive but have a limited life and store less energy per pound than other types.

Bharat Automotive Series
It is important to note that nearly all of the batteries commonly used in deep cycle applications are Lead-Acid. This includes the standard flooded (wet) batteries, gelled, and AGM. They all use the same chemistry, although the actual construction of the plates etc can vary considerably. Ni-Cads, Nickel-Iron, and other types are found in some systems, but are not common due to their expense and/or poor efficiency.

Bharat Power Back-Up Series
